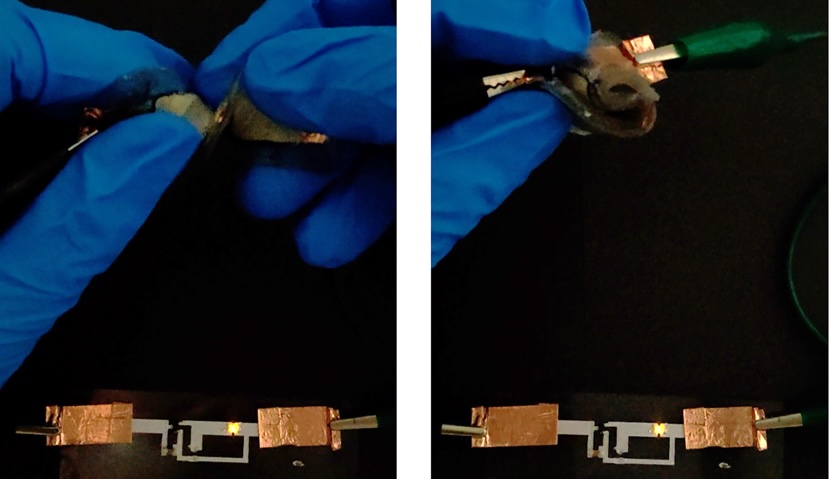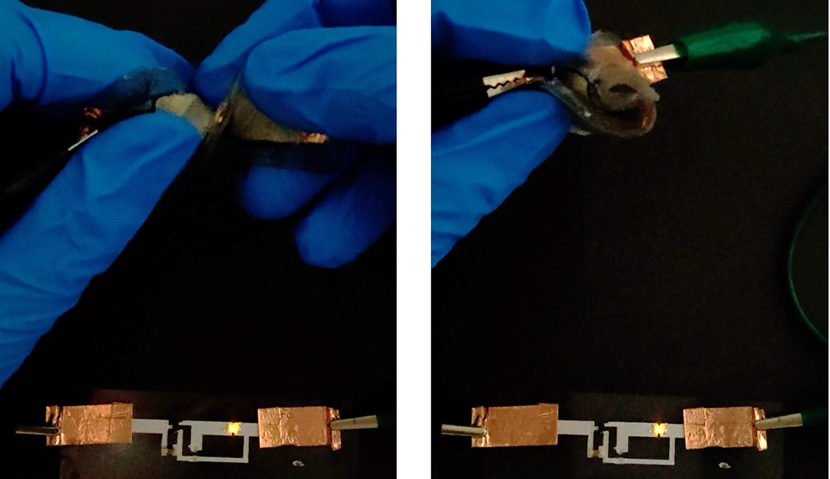
Le -ion batteries that strengthen everything from smartphones to electric cars are usually packed in strict, sealed walls that prevent pressure from damaging their ingredients and prevents air from getting into contact with their firearms and toxic electrolytes. It is difficult to use such batteries such as soft robots or wear, so a team of scientists from the University of California, Berkeley created a flexible, non -toxic, jelly battery that can survive by bending, rotating and even cutting with razors.
Although the flexible batteries using hydrogel electrolytes have already been achieved, they have brought significant defects. “All such batteries can be [only] Work [for] Levy Lin, a mechanical engineering professor at UC Berkeley and senior author of the study, says Levy Lin, a little time, sometimes a few hours, sometimes a few days, “
Lightning in the water
“Current -era batteries need a strict package because the electrolyte they use is explosive, and what we wanted to make was a battery that would be safe to work without this hard package,” Lin told RS. Unfortunately, elastic packaging made of polymer or other stretch substances can easily enter air or water, which will react with standard electrolytes, which will cause a lot of heat, which can result in fire and explosion. That is why, in 2017, scientists began experimenting with the Solid State Hydrogel Electrolytes.
These hydrogels were made of a polymer net that gave them their shape, borex or hydrogen bonds such as cross -linkers that placed this net together, a liquid phase made of water, and salt or other electrolyte additional ions that pass through the water gel because the battery is charged or charged.
But such hydrogels had a fair share of the problems. The first was a tight electrochemical stability window. A secure zone of voltage can be exposed. “It is really limited how much voltage your battery can do out,” says Paishing He, a researcher at the UC Berkeley sensor and activator center and the main author of the study. “Nowadays, batteries usually run on 3.3 volts, so their stability window should be higher, maybe four volts, something like this.” Water, which was the basis of these hydrogel electrolytes, usually breaks into hydrogen and oxygen when approximately 1.2 volts appear. The problem was solved by using highly concentrated salt water filled with highly Florinated lithium salts, which reduces the chances of being broken. But because of this, the researchers were directly involved in protective issues, as Florinated lithium salt is extremely toxic to humans.